3-step Staircase: CAR T-cell Therapy
January 28, 2020
Hello everyone! The Noctiluca is excited to present a new series to our readers called “3-Step Staircase.” In this weekly series, I will present contemporary scientific literature at three levels of understanding in the hopes of contributing to a more scientifically-versed school community. First, I will discuss the topic in layperson’s terms, next, at the level of an AP-science course, and finally, at the level of a research scientist in the field. By discussing advanced scientific topics at increasing levels of understanding, I hope this series will appeal to a large audience, where readers are encouraged to tailor their reading to their own levels of scientific literacy. Moreover, I hope this series will serve to inspire and excite the next generation of scientists within the North community by providing them with a window to the world of scientific research. For our first article of the series, we will examine CAR T-cell therapy, which is a rapidly advancing area of research.
Level 1: Layperson
Cancer, autoimmune diseases, infections, arthritis, and even allergies. Such afflictions have continued to plague humanity, and it has been up to scientists and researchers worldwide to persistently search for cures. One recently developed approach could potentially be the solution to many of these problems, most notably cancer: Chimeric Antigen Receptor (CAR) T-cell therapy. But don’t let the name scare you. CAR T-cell therapy is a form of immunotherapy; in other words, it works by helping the body’s own immune system to fight a disease as opposed to introducing a foreign drug, and it has been widely applied to treat a variety of cancers.
In our bodies, immune cells recognize abnormal cells by monitoring a group of molecules found on the surface of all body cells, called antigens. Such “flags” on cell surfaces allow the immune system to closely inspect potential problems and even decide whether to launch an attack. In the very early stages of cancer, our immune cells can actually easily kill individual cancerous cells as they arise; however, as the cancer progresses, it can develop new strategies to evade detection by the immune system. Essentially, our immune system is rendered unable to even detect the cancerous cells anymore, but CAR T-cell therapy allows us to manually guide our body’s own immune cells back to such cancerous cells. We can literally program our own immune cells to effectively attack any disease-causing cells with high specificity, making this one of the most innovative cancer treatments that holds remarkable potential for future success.
Level 2: AP Science
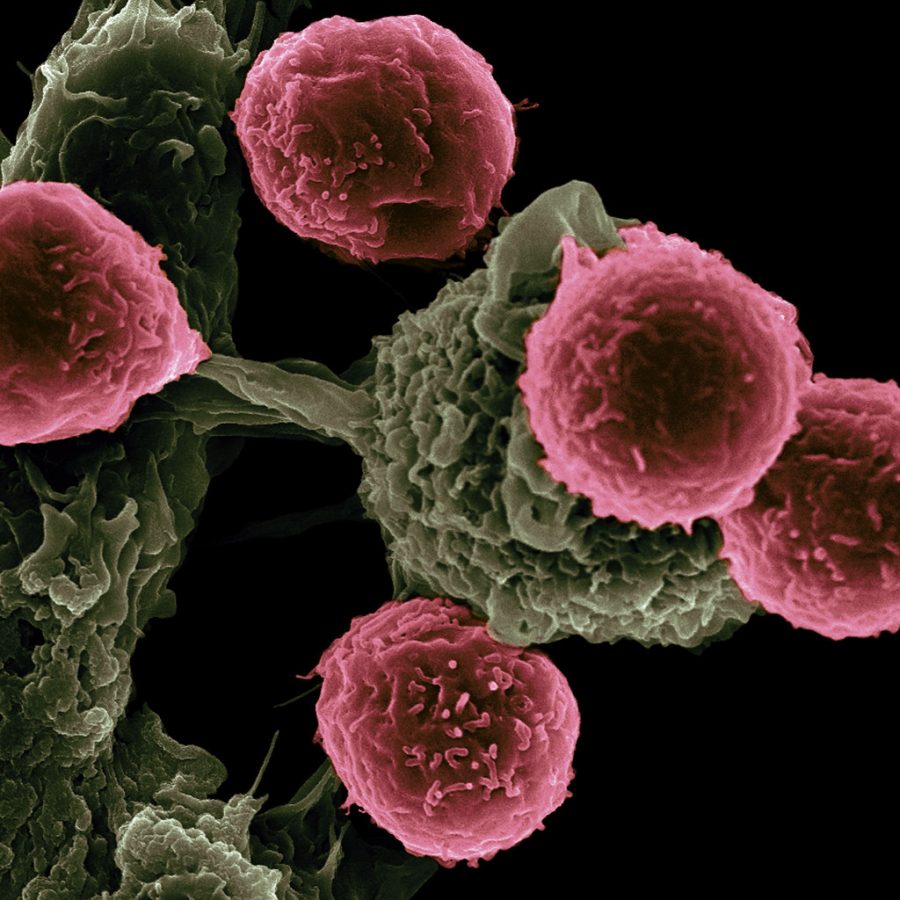
As one of the leading causes of death worldwide, cancer has continued to be at the focus of research with billions of dollars in funding poured into finding innovative treatments. The heterogeneity of cancerous cells has limited the effects of a majority of such treatments, and researchers are constantly searching for more effective therapeutic approaches, such as immunotherapy, which utilizes and stimulates the body’s immune system to help it fight against a disease. One of the most innovative approaches, Chimeric Antigen Receptor (CAR) T-cell therapy, utilizes our own body’s T-cells to detect and attack cancerous cells. As lymphocytes that migrate from the bone marrow to the thymus, T-cells are the cells of our immune system which search for and destroy pathogens in the body, including cancerous ones, by binding to antigens on cell surfaces. However, as cancerous cells continue to mutate, they begin to develop new mechanisms to evade detection by the immune system, thereby rendering our bodies incapable of fighting cancer without aid.
CAR T-cell therapy enables the re-detection and attack of such cancerous cells. The process begins with collecting blood from a patient and isolating their T-cells. Next, researchers are actually able to genetically engineer that patient’s T-cells to begin producing synthetic receptors on their surface called chimeric antigen receptors, or CARs. Such receptors act as guides that allow the T-cells to begin detecting antigens on the surface of tumor cells, which were previously masked from the immune system. After engineering the patient’s T-cells to actually begin self-producing a CAR that is specific to the antigens of their cancerous cells, the T-cells are re-injected into the patient, where they will further multiply and attack cancerous cells.
While CAR T-cell therapy has revolutionized the field of oncology, it continues to open more doors to a wide variety of applications in public health beyond only cancer. The specificity associated with CAR T-cell therapy enables its use in treating autoimmune disorders, such as lupus, or even viral infections, including HIV and Hepatitis C. The FDA has recently approved CAR T-cell therapy for adults with specific lymphoma types and for children or young adults with acute lymphoblastic leukemia. Hundreds of clinical trials are currently being run using CAR T-cell therapy with blood cancer, and researchers are even starting to employ this therapy for use with solid tumors. The future holds great potential for CAR T-cell therapy.
Level 3: Research Scientist
As a leading cause of death worldwide, cancer has been an area of particular focus for scientists across the globe. The immune system is designed to functionally control tumor development; however, as cancer progresses, cancerous cells often evolve to avoid tumoricidal attack. In the early stages of tumor development, cytotoxic immune cells such as natural killer and CD8+ T cells identify and eradicate the most immunogenic cancer cells, namely those that are most able to produce an immune response. This eliminates and selects for cancer cells that are less immunogenic and invisible to detection by the immune cells.
With billions of dollars in funding annually pouring into developing treatments for cancer, several conventional cytotoxic approaches for neoplastic diseases have been developed. However, the heterogeneity of the tumor microenvironment has limited the effectiveness of such drugs, shifting the focus toward immunotherapy, which enhances a patient’s immune system to target and attack cancerous cells. The ability to actually reprogram our own immune cells to fight against cancer has excited and compelled researchers worldwide.
In Chimeric Antigen Receptor (CAR) T-cell therapy, a patient’s T-cells are extracted in a process called leukapheresis and genetically modified to express a CAR that is specific to the antigen of their tumor cells. After ex vivo cell expansion, the modified T-cells are re-injected into the patient, where they can begin targeting cancerous cells.
The external domain of a CAR is engineered, either through viral-based gene transfer methods or nonviral methods (i.e. CRISPR/Cas9 technology), to detect a specific antigen on a tumor cell. Recognition of cancerous cells by this modified T-cell activates the internal signaling domain of the molecule and prompts the T-cell to begin attacking the cancer cell. There are even several varieties of CARs, which have multiple internal domains that further strengthen an immune response against a programmed target.
However, CAR T-cells may sometimes result in unexpected toxicities including: cytokine release syndrome, neurologic toxicity, “on target/off tumor” recognition, and anaphylaxis. Getting rid of such toxicities is crucial to the ultimate success of this burgeoning technology.
Further Reading…
https://www.annualreviews.org/doi/abs/10.1146/annurev-med-062315-120245
https://www.frontiersin.org/articles/10.3389/fimmu.2019.02711/full
https://www.sciencedirect.com/science/article/pii/S2372770516300353
https://jhoonline.biomedcentral.com/articles/10.1186/s13045-017-0423-1